Ripple Therapeutics Presents at ARVO 2024
Ocular tolerability and IOP-lowering evaluation of the RTC-620 intracameral implant in normotensive beagle dogs
Ike Iqbal K Ahmed, Kyle Battiston, Shadi Taghavi, Mahta Massoud, Eamon Kelly, Hans Fischer, Dimitra Louka, Matthew Statham, Jonathan Day, Adam Daley, Ian Parrag, Wendy Naimark
IBE-814 IVT Implants reduce treatment burden in subjects with DME and RVO due to sustained-release of dexamethasone: An analysis of the First-in-Human Phase 2 RIPPLE-1 trial
Kelli Wootton, Gillian Mackey, Madeline Simpson, Joseph Reiz, Wendy Naimark

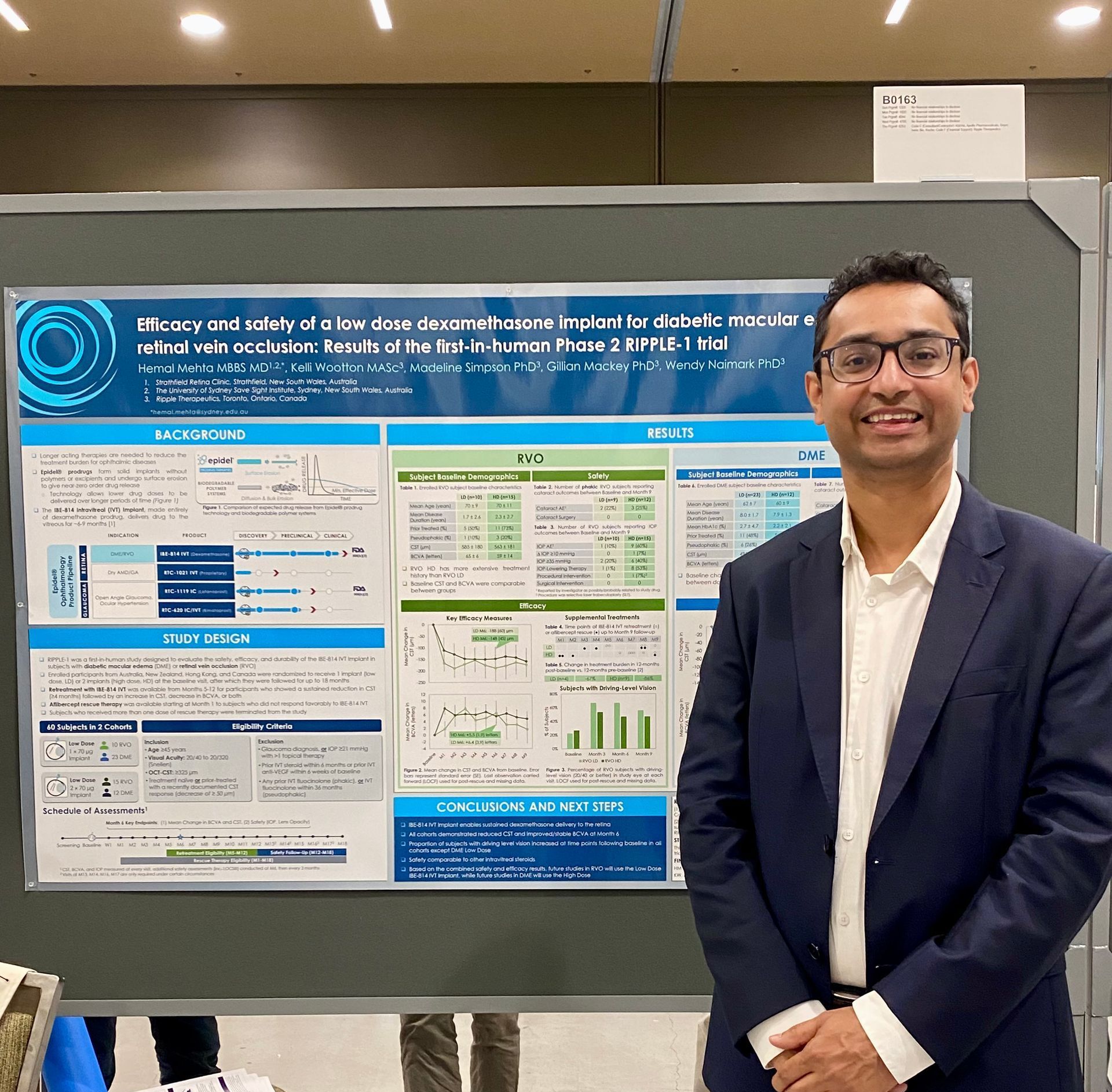
Efficacy and safety of a low dose dexamethasone implant for diabetic macular edema and retinal vein occlusion: Results of the First-In-Human Phase 2 RIPPLE-1 trial
Hemal Mehta, Kelli Wootton, Madeline Simpson, Gillian Mackey, Wendy Naimark
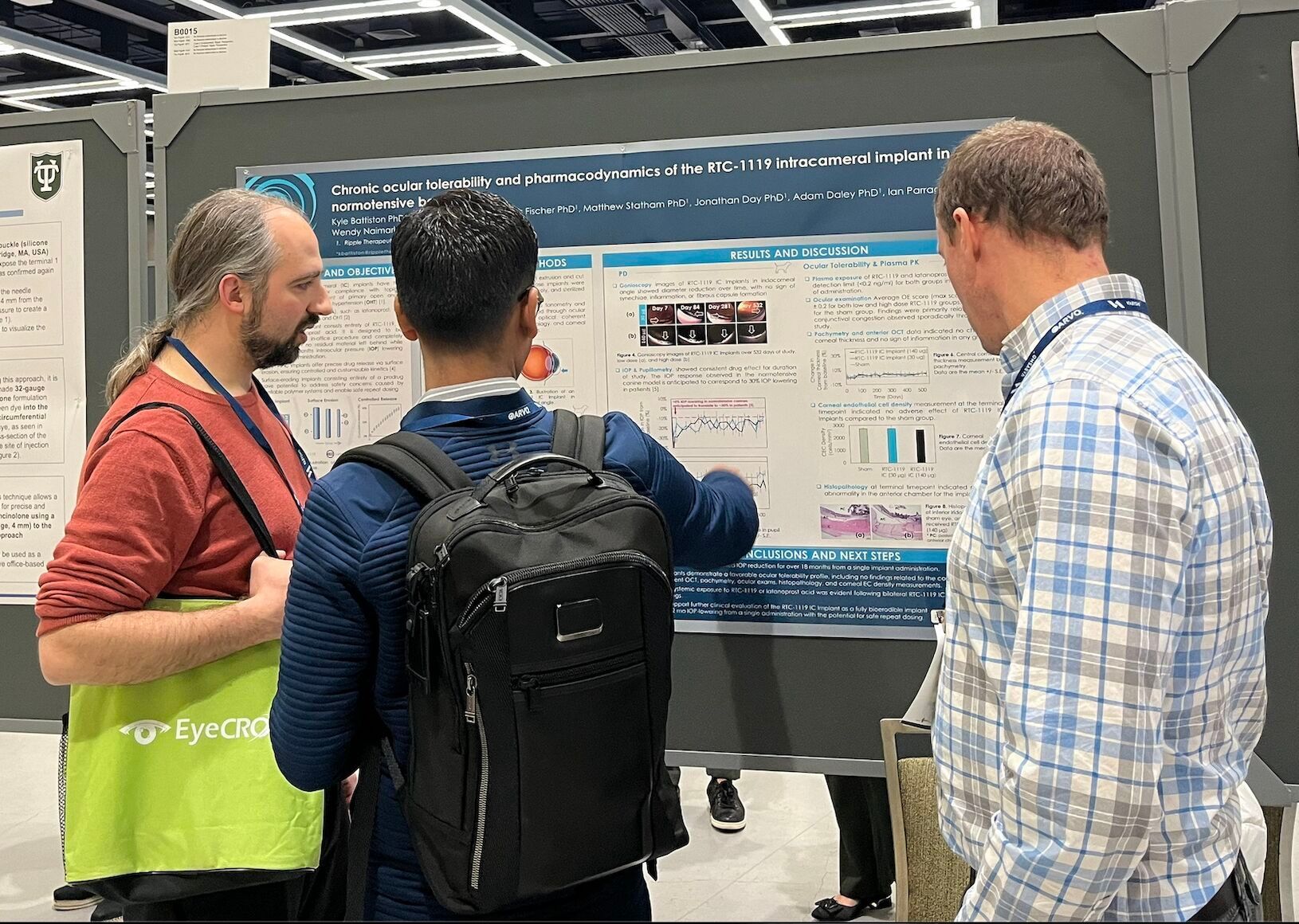
Chronic ocular tolerability and pharmacodynamics of the RTC-1119 intracameral implant in normotensive beagle dogs
Kyle Battiston, Shadi Taghavi, Hans Fischer, Matthew Statham, Jonathan Day, Emily Baldwin, Adam Daley, Ian Parrag, Wendy Naimark
TORONTO, CANADA / ACCESSWIRE / October 15, 2024 - Ripple Therapeutics Corporation, a clinical stage company focused on improving ophthalmic therapeutics with controllable sustained delivery implants, is pleased to announce evaluation and licensing agreements with Glaukos Corporation (NYSE: GKOS), an ophthalmic pharmaceutical and medical technology company focused on novel therapies for the treatment of glaucoma, corneal disorders and retinal disease. Ripple’s patented technology platform is based on a discovery that drugs can be chemically engineered into controlled release pharmaceuticals without the use of polymers or excipients. These proprietary prodrugs undergo surface erosion to give zero order release kinetics and are highly engineerable to tailor both drug dose and duration. The advantages of this technology include lower molecular weight and higher drug loading allowing for smaller implants and a lack of degradation products which provides both a clearer regulatory path as well as an improved safety profile. With an extended duration of therapeutic benefit, this technology will also reduce the treatment burden for patients. These agreements enable Glaukos to leverage Ripple’s proprietary technology platform to create sustained release implants of targeted APIs for both glaucoma and retinal diseases. If the program is successful, the evaluation agreement will automatically convert into a licensing agreement with future milestone payments and royalties. “We believe Ripple has one of the most promising drug delivery technologies currently under development,” commented Tomas Navratil, PhD, Chief Development Officer, Glaukos. “We are pleased with the progress of our collaboration and have enjoyed working with the Ripple team as we work together to bring these much-needed sustained release products to patients with critical unmet needs.” “This is the first of what we believe will be a number of transactions using our technology platform in concert with partners’ APIs to create sustained release implants which will benefit patients with extended duration and improved safety”, commented Tom Reeves, President & CEO, Ripple Therapeutics. “We look forward to continued collaboration with the entire Glaukos team.” About Ripple Therapeutics Ripple Therapeutics Corporation is a privately held clinical stage company focused on improving ophthalmic therapeutics with controllable sustained delivery implants. Ripple’s patented technology platform is based on a discovery that drugs can be chemically engineered into controlled release pharmaceuticals without the use of polymers or excipients. These proprietary prodrugs undergo surface erosion to give zero order release kinetics and are highly engineerable to tailor both drug dose and duration. The advantages of this technology include lower molecular weight and higher drug loading allowing for smaller implants and a lack of degradation products which provides both a clearer regulatory path as well as an improved safety profile. With an extended duration of therapeutic benefit, this technology will also reduce the treatment burden for patients. www.rippletherapeutics.com Media Contact (Ripple) Julie Fotheringham, V.P. Marketing, People & Culture, Ripple Therapeutics M: 416-951-7988 E: jfotheringham@rippletherapeutics.com


